Immunohistochemistry
Immunohistochemistry (IHC) is a microscope-based imaging technique that allows for the visualization of cell components - such as proteins - within a tissue sample. IHC works by using antibodies that are conjugated to enzymes which form detectable compounds, allowing identification of specific antigens.
IHC is a useful technique because it is both quantitative and qualitative, allowing for information that can be used to determine the distribution and localization of cell components within sample cells or tissues.
The purpose of IHC is to identify the location of specific antigens within a tissue sample, which makes it an important diagnostic tool to identify and study tumours, cancers, and infectious diseases.
Immunohistochemistry Resources
View some of our additional resources in regards to an immunohistochemistry overview and testing methodologies.
- An Introduction to Multiplex Immunohistochemistry
- Practical Overview of Multiplex Immunohistochemistry using TSA
- Benefits of Fluorescent Multiplex Immunohistochemistry using Tyramide Signal Amplification
- Retrieval buffers: Citrate vs. Tris-EDTA
What is the Purpose of IHC?
IHC is an important technique used in healthcare and pathology to analyze molecules of interest, in relation to both healthy and diseased tissues.
Why Use IHC?
IHC techniques are popular in how they detect and analyze protein expression without destroying the composition of the sample. Maintaining this composition is important when diagnosing any cell abnormalities with respect to the structure of cells and surrounding tissues.
How Does IHC Work?
IHC is a type of immunostaining technique based on the specific recognition of a target antigen by an antibody.
To begin, a 4-7µm thick tissue section is affixed to a glass slide. In order to prevent non-specific staining, the slide is blocked with a buffer containing irrelevant proteins, before one or more antibodies specific to the target proteins of interest are incubated on the slide.
Signal development in IHC is based on enzymatic reactions leading to the deposition of a colored precipitate onto the slide.
Either an enzyme-conjugated primary antibody or a primary-secondary pair can be used; the most commonly used enzymes in IHC being horseradish peroxidase (HRP) or alkaline phosphatase (AP).
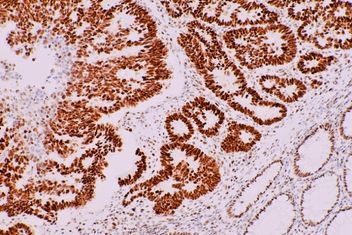
Different substrates produce different colored stains; the most commonly observed colors in IHC stains include brown (substrate, DAB) and red (substrate, AEC). The limit to the number of proteins that can be marked on a single slide by IHC is limited only by the number of differently colored enzyme-substrate complexes available; typically, IHC is used to visualize one or two proteins.
After the enzyme-substrate reaction has taken place, the slide is counterstained with hematoxylin, a blue stain that marks cell nuclei. IHC slides can be viewed by light microscopes at a low magnification to view protein distribution over an entire section, or at a high magnification to analyze specific cells or tissue regions.
2-Step Protocols
See the links below regarding protocols of common IHC testing methodologies.
Immunoperoxidase Protocols
- FFPE tissues and cell blocks
- Epitope Retrieval Method for FFPE tissues and cell block
- Formaldehyde-Fixed Cells and Cytospin Preparations
- Cells Grown in Culture and Cytospin Preparations
Immunofluorescence Protocols
- FFPE tissues and cell blocks
- Epitope Retrieval Method for FFPE tissues and cell blocks
- Formaldehyde-Fixed Cells and Cytospin Preparations
- Fresh Frozen Tissue Sections
- Cells Grown in Culture and Cytospin Preparations
Related Applications
Immunohistochemistry FAQs
All IHC Antibodies are tested on tissue microarrays.
Yes, with immunoperoxidase (DAB) methods you have a permanent slide representing your data. The surrounding tissue is visible to further enhance your data. Also, autofluorescence can confound your data.
IF is appropriate for Immunocytochemistry (ICC).
Refer to the individual IHC-validate antibody data sheet. All IHC-validated antibody data sheets have images demonstrating positive staining.
Formalin-fixed, paraffin-embedded tissues should be fixed in formaldehyde no longer than 24 hours. Tissues should not be thicker than 3mm to allow fixative penetration.
By clicking “Acknowledge”, you consent to our website's use of cookies to give you the most relevant experience by remembering your preferences and to analyze our website traffic.
